Managing Adult Malnutrition
Managing Adult Malnutrition
Including a pathway for the appropriate use of
oral nutritional supplements (ONS)
Managing frailty, sarcopenia & malnutrition
Including Assessment and Actions to Improve Outcomes and Quality of Life
Written by Dr Sanjay Suman, Consultant Geriatrician and Dr Anne Holdoway, Consultant Dietitian.
FRAILTY PATIENT & CARER INFORMATION SHEET
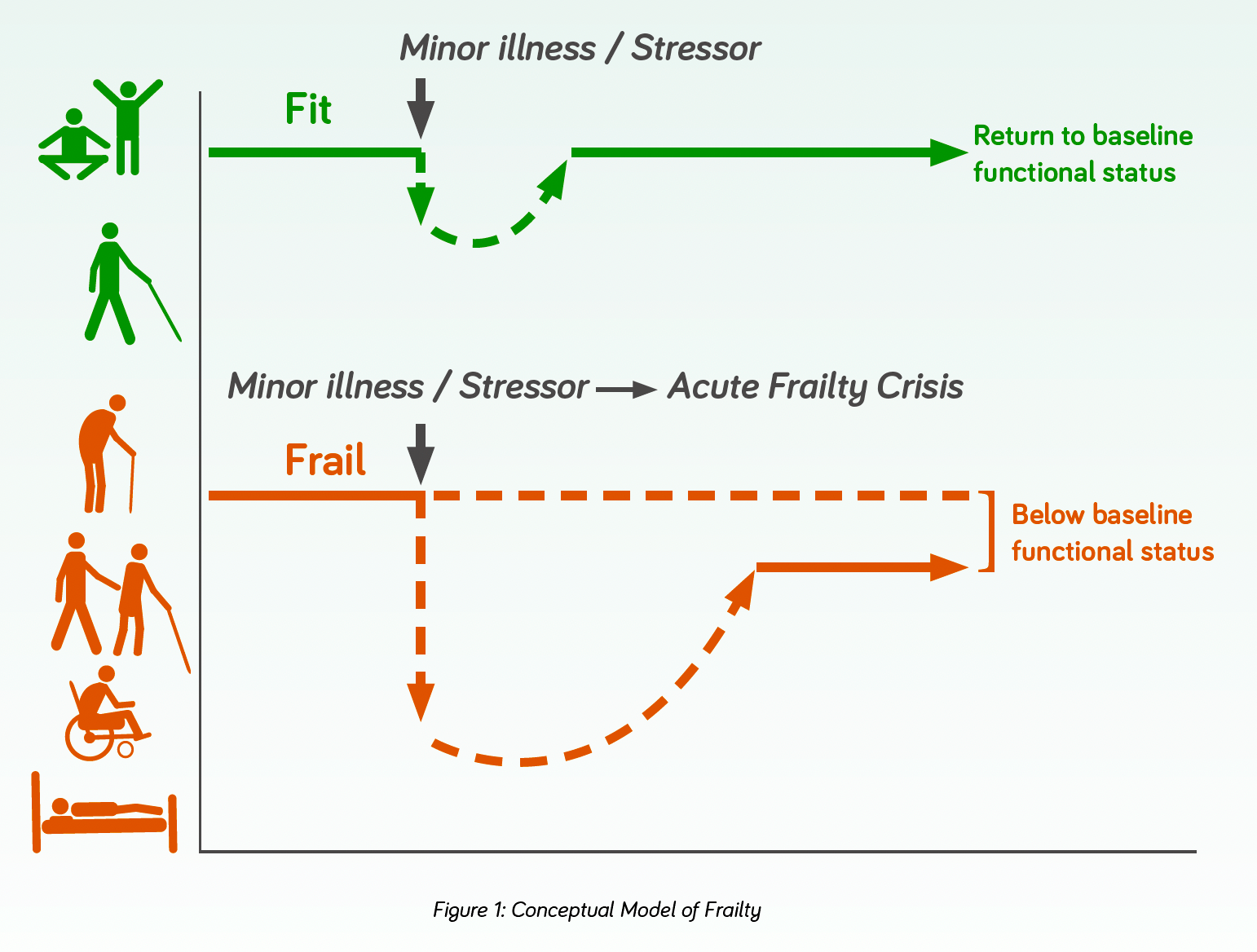
- Frailty is a state of vulnerability characterised by a loss of reserve across multiple organ systems. In simple terms, someone who is frail is less able to “bounce back” after a stressor event, be that something minor like a urine infection or a more significant event like a fractured hip (figure 1).
- The event can trigger major changes in health from which the patient may fail to return to their previous level of health1.
- The decline in physiologic reserve and function across multi-organ systems contributes to adverse health outcomes2.
- Frailty is frequently associated with ageing; however, it can also arise with many progressive long-term conditions and can occur in younger people who have illness or disease3-5.
- It is often considered a progressive long-term condition, with episodic deteriorations.
People who are frail usually have three or more of five symptoms that often occur together. These include:
- Weight Loss - Unintentional weight loss (5 or more kilograms within the past year) or decreased appetite.
- Fatigue - Feeling overtired, with low energy and a strong desire to sleep that interferes with normal daily activities.
- Loss of Strength - Muscle loss and weakness, low grip strength measured with hand-grip dynamometer.
- Slow Walking Speed - Walking at a slow space or measured slow gait, reduced arm swing bilaterally.
- Low Activity Level - Low frequency of moderate intensity activity.
(Adapted from the Phenotypic Model6)
FRAILTY – FACTS AND FIGURES
- An estimated 1.8 million people in the UK aged 60 and over are living with frailty7
- All older adults are at risk of developing frailty. There are currently 11 million people in the UK > 65 years of age8. 35% of people > 65 years are living with some degree of frailty9, equivalent to 4.5 million people8
- Approximately 47% of hospital inpatients aged over 65 are affected by frailty9
- Risk levels are substantially higher among those with comorbidities.
69% of people >85 years live with multiple conditions9. Low socioeconomic status, poor diet, and sedentary lifestyles are also risk factors10
The prevalence of frailty is expected to rise as people live longer, often with multiple long-term conditions11. In the absence of intervention, the increasing numbers of people affected by frailty will have a significant impact on health and care requirements and provision9.
Having frailty places a person at increased risk of a number of adverse outcomes, including reduced mobility, falls, fragility fractures due to a high prevalence of underlying osteoporosis, disability, acute confusion, incontinence, hospitalisation, and premature death1,12.
People living with frailty are less able to respond to stress factors such as acute illness, injury or changes in their environment including personal or social circumstances. Even a seemingly minor health event, such as a simple virus for example, can result in adverse health outcomes and loss of independence13. As well as the cost to the individual’s health and well-being, frailty costs the UK healthcare system around £5.8 billion a year9. Frailty is, however, amenable to interventions. Timely medical, nutritional and physical interventions that address the underlying causes can avoid unnecessary harm, improve outcomes and an individual’s (and their families) lived experience1. Interventions rely on an understanding of several key components - sarcopenia (muscle strength) and nutrition are particularly relevant as they are modifiable factors.
Key Point
Encouraging people to stay well for as long as possible by keeping well nourished, active and managing long-term conditions, can reduce pressure on the wider health and care system, facilitate patient flow by getting individuals home after a hospital stay, and maintain independence and quality of life.
The concept of frailty and diagnostic criteria for sarcopenia and malnutrition continues to evolve. Malnutrition, frailty and sarcopenia affects a large proportion (~25%) of community dwelling adults14. Unintended weight loss, slow gait speed, low energy expenditure, self-reported exhaustion and poor grip strength are all phenotypically associated with frailty but are equally present in sarcopenia (a generalised loss of muscle strength and muscle function) and in malnutrition15.
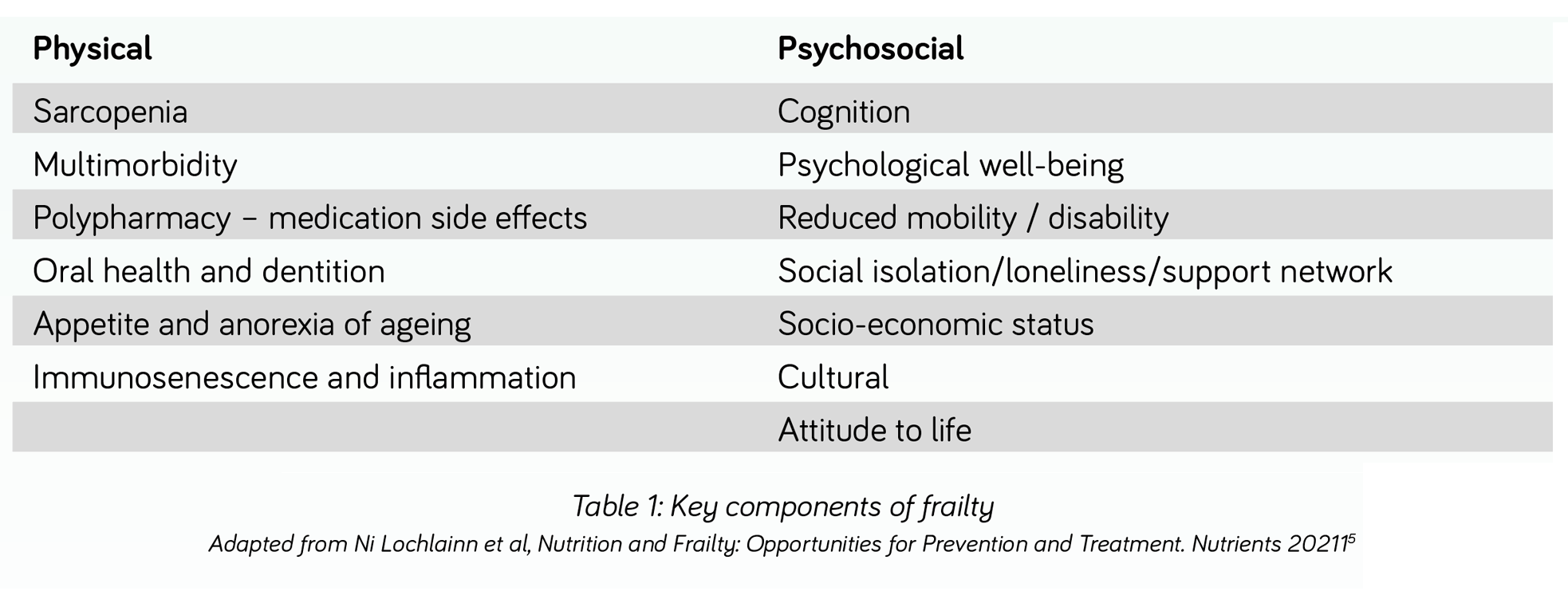
Table 1 illustrates key components of frailty many of which are relevant to sarcopenia and malnutrition16.
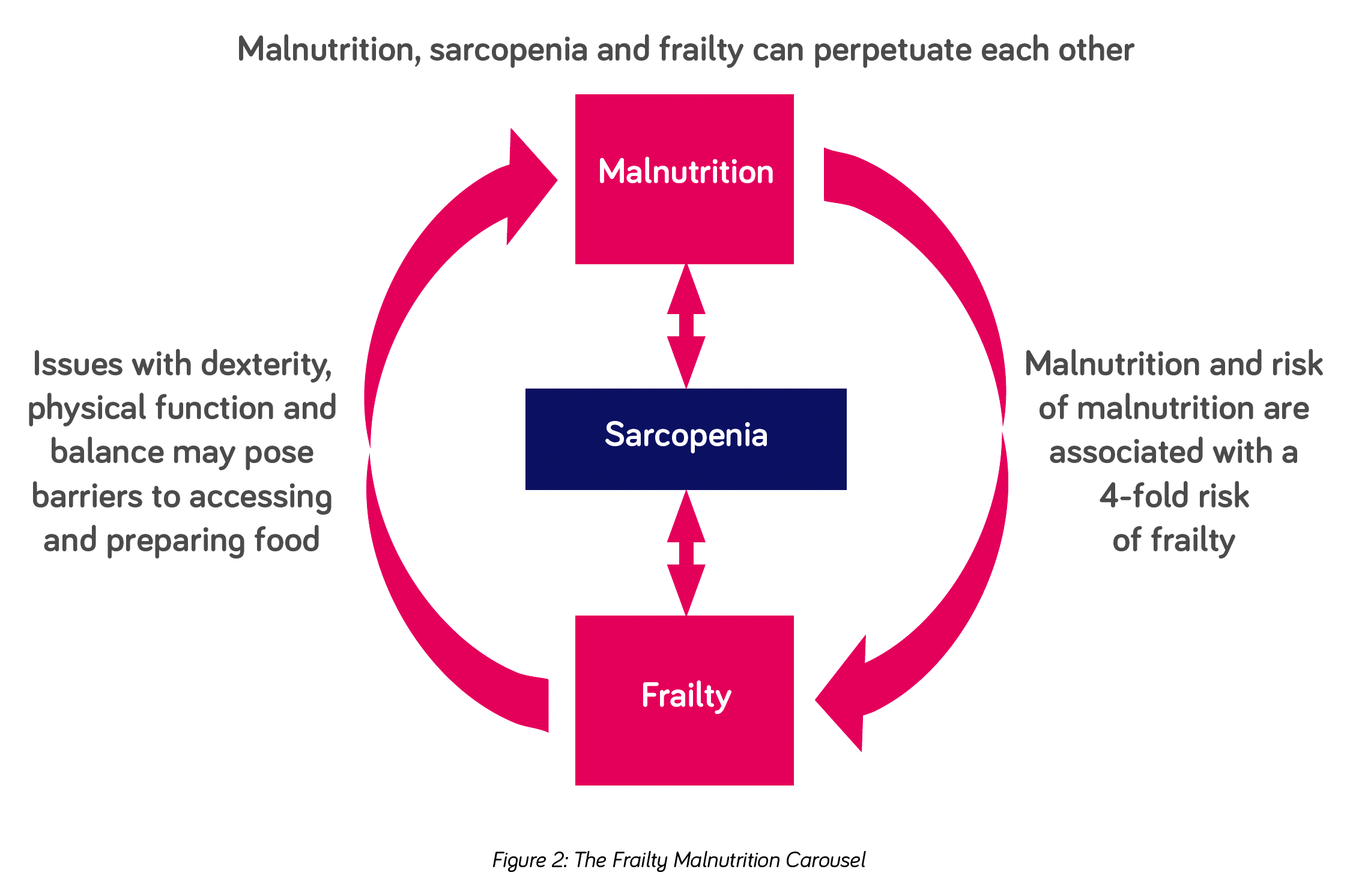
Just as the likelihood of frailty increases with increasing age, acute and chronic, progressive diseases and hospitalisation, so does malnutrition and sarcopenia17. There is considerable overlap between the three syndromes and one can perpetuate the other (see figure 2).
SARCOPENIA* is common among adults of older age but can also occur earlier in life. Disease, inactivity, and poor nutrition can all contribute17. Whilst sarcopenia can arise in the absence of malnutrition from muscle disuse or a lack of muscle stimulation (e.g. as a result of acute or progressive disease, bed rest, immobility), individuals with malnutrition have been found to have approximately three to four times the risk of developing sarcopenia than those without malnutrition18,19.
MALNUTRITION** plays a key role in the pathogenesis of sarcopenia and frailty and the prevalence of malnutrition increases with increasing severity of frailty20. Despite its prevalence and link to frailty, malnutrition continues to be under-reported and under-treated in primary care21. In addition, weight loss is still mistakenly perceived as an inevitable part of ageing or disease and therefore even if identified may go untreated.
As poor nutritional status is considered a key contributory factor influencing the development of frailty, strategies to treat and prevent frailty should consider the management of nutrition as a modifiable factor to be addressed and managed16,22. Unplanned weight loss, poor appetite, loss of interest in food, eating and drinking difficulties, diminished access to food, or the lack of ability or motivation to access food, prepare it and cook, are all hallmarks of an increased risk of malnutrition. All are potentially amenable to intervention according to an individual’s circumstances.
Although malnutrition and sarcopenia are often associated with low body weight, they are increasingly observed in adults aged 65 years and over, who are overweight or obese19. With two thirds of the UK adult population now overweight or obese23, malnutrition and sarcopenia arising from disease and poor nutritional intake, may be masked by a high body mass index (BMI). Care must therefore be taken to ensure that malnutrition and sarcopenia are not missed in these individuals.
*Sarcopenia - A syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength, resulting in reduced physical performance that can contribute to frailty, prolonged physical disability, increased risk of falls, a poorer quality of life and death
**Malnutrition – A deficiency of energy, protein and other nutrients that causes adverse effects on the body (shape, size and composition), the way it functions and clinical outcomes
Key point:
In clinical practice, given the overlapping nature of nutrition, muscle strength and frailty, if one finds frailty, one should look for malnutrition or sarcopenia, if one finds malnutrition one should look for frailty and sarcopenia and if sarcopenia is identified one should look for malnutrition and frailty.
FRAILTY
The identification of frailty can be both opportunistic - assessing for frailty in people who present to health and care services, or population-based where a more systematic approach is taken to proactively identify people who might be living with the condition.
FRAILTY IDENTIFICATION TOOLS
Identifying frailty, especially in older adults, typically involves using a variety of tools and assessments. These tools evaluate physical, cognitive, and psychosocial factors to determine a person’s vulnerability to health decline and predict the risk of adverse outcomes such as falls, disability, loss of independence, hospitalisation, and mortality. Furthermore, they assist in tailoring care and providing targeted interventions for at-risk individuals24.
A number of validated tools have been published over the years to identify frailty in an objective and standardised way.
Fried Frailty Phenotype (FFP): This tool identifies frailty based on five criteria:
Clinical Frailty Scale (CFS): This is one of the most commonly used tools and gives a score ranging from 1 (very fit) to 9 (terminally ill), assessing a person’s overall fitness and frailty based on clinical judgment of their health, energy, and activity levels25. It is user friendly and quick to apply in a variety of clinical setting across primary and secondary care. It requires minimum training for use by clinical and allied healthcare staff26. CFS also allows for scoring frailty in a patient with dementia with the degree of frailty corresponding to the degree of dementia. The scale facilitates communication across healthcare teams by providing a standardised framework for discussing frailty, tailoring interventions to prevent adverse outcomes and personalised care planning. A CFS app has been designed and made available to download free for smart devices.
Electronic Frailty Index (eFI): This is a population-based risk stratification tool embedded in Electronic Health Records used in primary care in England to support early identification of frailty. The eFI uses routine health record data to automatically calculate a score which can identify whether a person is likely to be fit or living with mild, moderate or severe frailty27. Early identification of frailty should lead to assessment and interventions in the community to promote independent living through reducing the risk of adverse outcomes such as falls, hospital admissions and adverse drug events related to polypharmacy.
Many other frailty assessment scales have been published over the years such as Edmonton Frailty Scale, PRISMA-7 Questionnaire, Geriatric 8 (G8) and Short Physical Performance Battery (SPPB). Once frailty is identified through any of these validated tools, a holistic multidisciplinary comprehensive assessment is necessary to facilitate individually tailored interventions.
SARCOPENIA
In the acute setting and specialist units skeletal muscle mass can be assessed by body composition methods such as CT scans, DEXA and bioimpedance28.
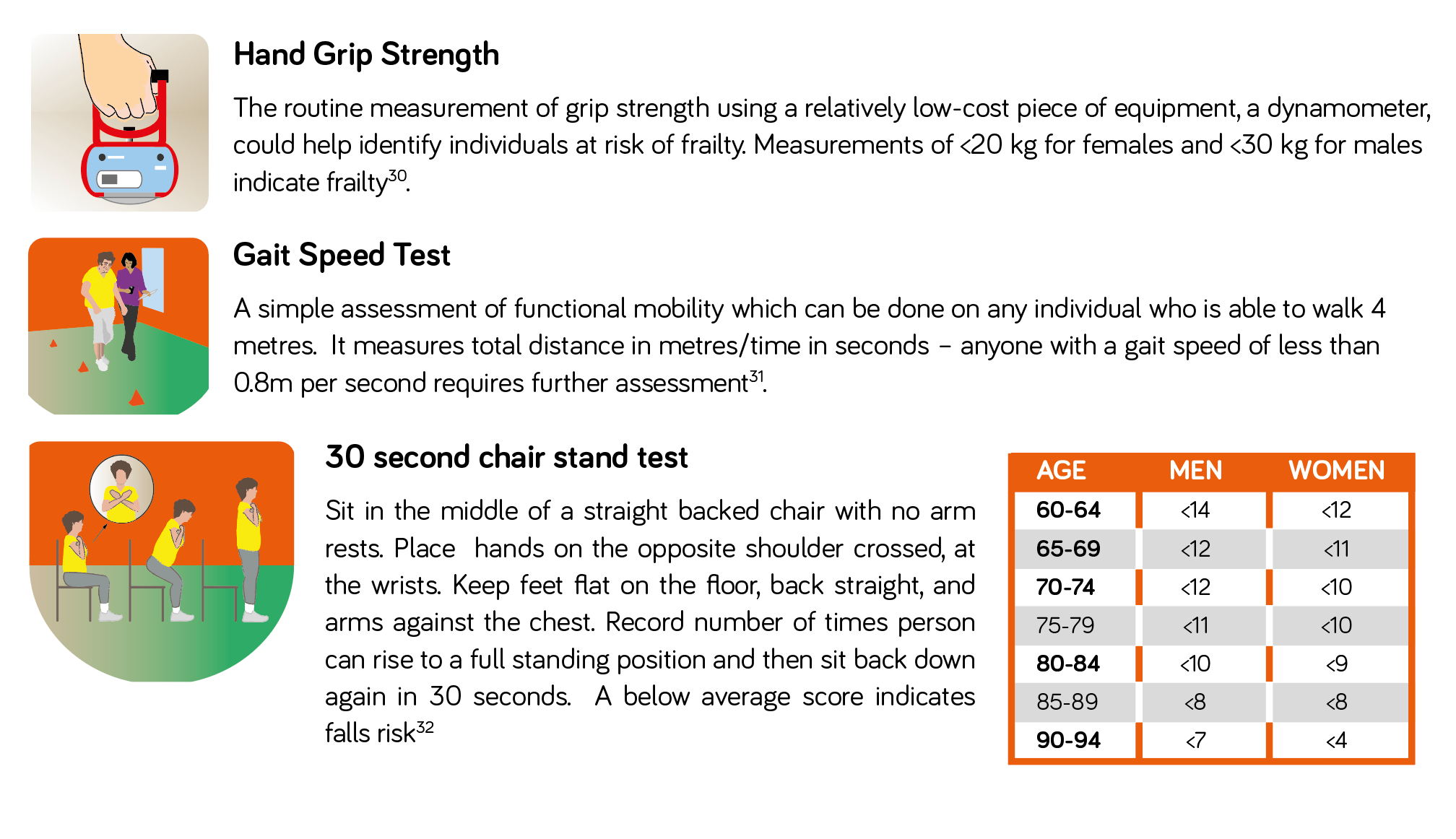
In community settings, practical evidence-based tools can be used such as the SARC-F questionnaire29 – a 5-item questionnaire to determine the likelihood of sarcopenia. A score of ≥4 suggests sarcopenia is likely. The SARC-F questionnaire should be followed by simple strength measurements, such as hand-grip strength and the 30 second chair stand test (for further information on these measures see box 1).
Functional Assessments to Diagnose Poor Muscle Health
All encounters with health and social care professionals provide the opportunity to assess for frailty in those who are vulnerable - simple clinical observations such as observing how an individual walks across the clinic room or gets up out of a chair can be a useful indicator of frailty. Hand grip dynamometry, the gait speed test and 30 second chair stand test are recommended as more formal assessments of muscle strength and function1.
Box 1: Measuring Muscle Strength
MALNUTRITION AS A POTENTIAL RISK FACTOR FOR THE CAUSE AND PROGRESSION OF FRAILTY
NICE recommends that a validated screening tool, such as the Malnutrition Universal Screening Tool (‘MUST’)33 is used to identify adults at risk of malnutrition, this combines assessment of BMI, recent unplanned weight loss and presence of acute illness. For patients who are digitally literate, use of the MALNUTRITION SELF-SCREENING website can be encouraged along with reporting of results direct to the community healthcare team e.g. GP surgery. The PATIENTS ASSOCIATION NUTRITION CHECKLIST is also a useful alternative to facilitate self-screening amongst individuals, involving family members if needed.
In individuals who are identified as malnourished, or at risk, undertaking further assessment to identify the issues interfering with the ability to eat and drink, and addressing those that can be reversed or modified, should be an integral component of the treatment plan34. For example:
- treatments and medications can have side effects which can impact on nutritional status, eating and drinking
- weakness and disability e.g. loss of dexterity, poor physical function and being unable to stand for a period, can have a detrimental effect on dietary intake and nutritional status by impacting on the patient’s ability to access and prepare food.
Figure 3 summarises the combined use of 'MUST' and SARC-F as a screen for malnutrition and sarcopenia and potential next steps to take based on risk. The Malnutrition Pathway MANAGING ADULT MALNUTRITION IN THE COMMUNITY document includes further advice on screening, assessment and management of malnutrition.
Figure 3: Addressing Malnutrition
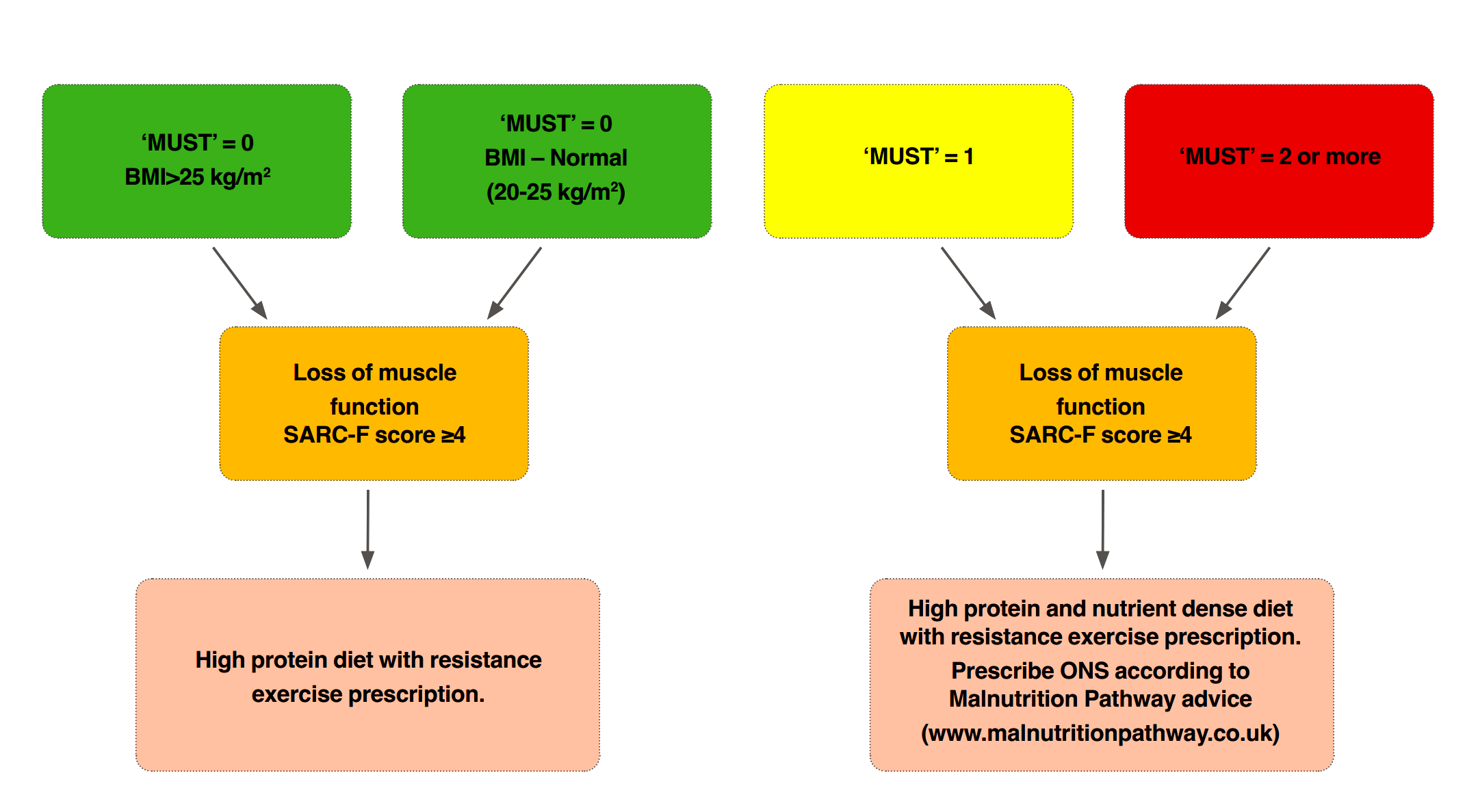
To reduce the clinical and economic burden of frailty, efficient, feasible, and cost-effective interventions to prevent or slow deterioration, maintain independence and maintain or improve quality of life35 are critical. Supporting people with frailty in the community including preventative actions, has the capacity to keep individuals in better health, preserve independence e.g. within the home and in familiar surroundings, for longer, subsequently reducing pressure on the wider health and care system and optimising patient flow e.g. discharge9.
Healthy ageing including good nutrition, keeping alcohol to within recommended limits if consumed, staying physically active and remaining engaged in the local community (avoiding loneliness) reduces the risk of developing frailty36.
Key Point
Frailty, sarcopenia and malnutrition should not be considered an inevitable part of ageing and disease, all are amenable to interventions which should include nutrition, exercise (activity) and review of medicines17,37.
The comprehensive geriatric assessment (CGA) is considered a vital framework for managing older people suspected of having frailty. The CGA seeks to evaluate areas of health and care needs that contribute to frailty and influence quality of life. Figure 4 outlines the approach to managing frailty using CGA.
The British Geriatrics Society has produced a CGA TOOLKIT FOR GPS38 and other healthcare professionals working in primary care settings, this explains the CGA in more detail, outlining what circumstances to use it in and how to co-ordinate planning and involvement of social services. It also includes guidance on specific medical issues that older patients may present with.
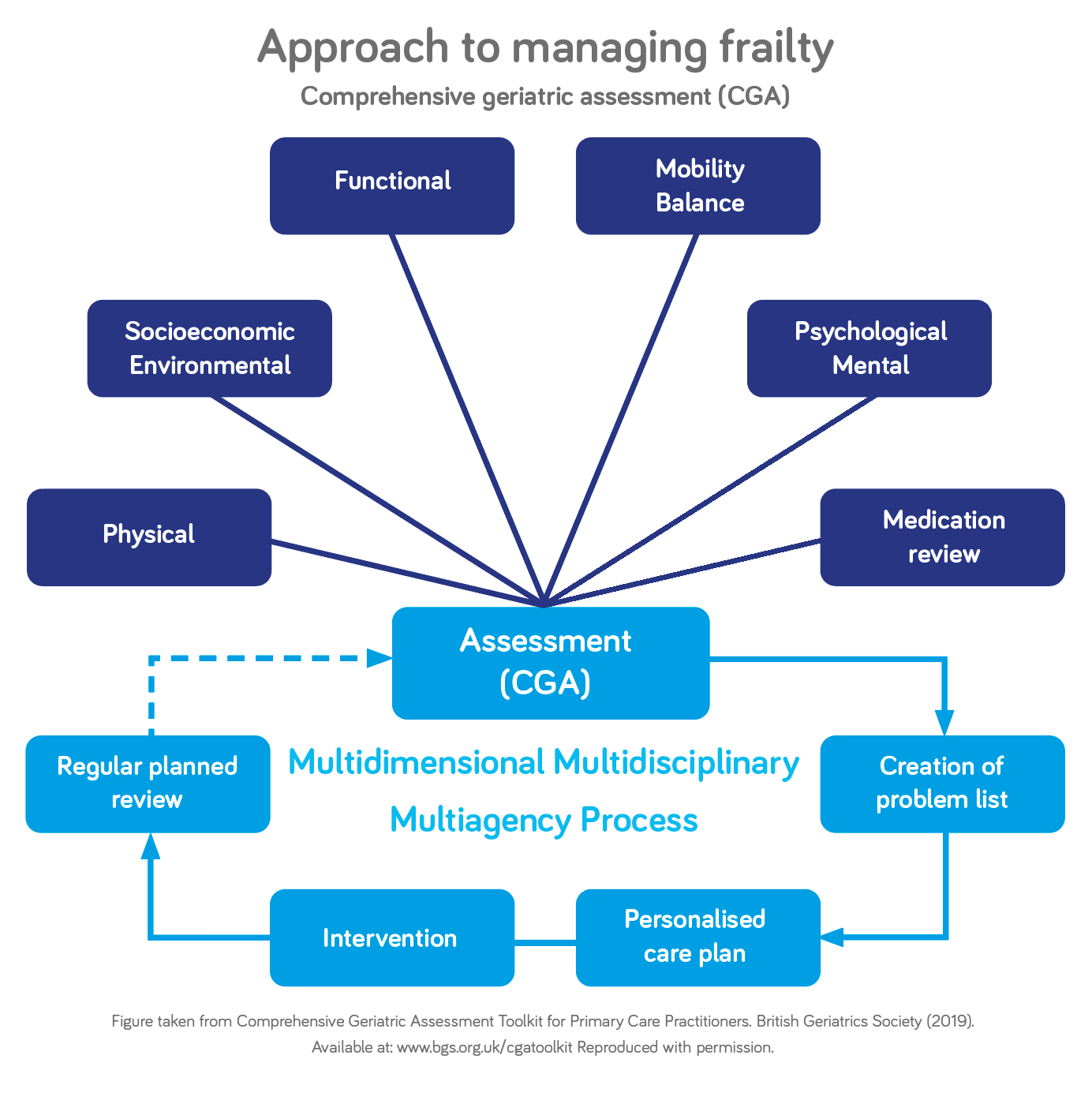
Figure 4: Approach to Managing Frailty using CGA
Exercise and nutrition remain a cornerstone of treatment for the management of malnutrition, sarcopenia and frailty. Exercise can help to preserve or improve muscle strength, balance, and endurance. Good nutrition is essential to support muscle strength and overall health and well-being. People living with, or at risk of frailty, will often need help and advice to ensure that they are getting enough calories, protein, and other essential nutrients. Research on physical exercise and nutritional interventions show promising effects on frailty status, functional outcomes, and cognitive outcomes35.
Immobility, poor nutrition and inflammation associated with disease, trigger and accelerate muscle loss31. Whilst historically body weight and nutritional intake have often been relied upon as outcome measures, this approach may fail to measure the real impact of interventions, including nutrition interventions, in combination with physical therapy, on functional outcomes and quality of life39. With the increasing evidence that nutrition and movement make a positive difference to health outcomes, it is important that muscle strength and function through optimal nutrition and physical exercise (particularly resistance activity) is considered. The synergistic effect of exercise and protein can facilitate the reversal of some elements of frailty. A combination of muscle strength training and protein supplementation has been found to be the most effective intervention to delay or reverse frailty and the easiest to implement in primary care40.
BUILDING MUSCLE: KEY NUTRIENTS - THE PROTEIN FACTOR
Muscles work to support the body and enable movement, they are important for mobility, balance, posture, strength and energy. Our muscles are in a constant state of turnover – being broken down and rebuilt (synthesised). Many factors influence muscle breakdown and synthesis including hormones, activity, nutrition, age, health and illness. As we age the body becomes less adept at using protein for muscle synthesis. During illness we also tend to break down our muscles to release essential amino acids to support the immune system. For this reason daily requirements for protein are higher as we age and in disease (see table 2)41,42.
PROTEIN QUALITY
The quality of the protein in our diet, or more specifically the amino acids which are the building blocks of protein, are a key determinant of the capacity for protein to influence muscle health43. The amino acid leucine and its derivative β-hydroxy-β-methylbutyrate (HMB) are receiving increasing attention in their role in muscle mass and function. Both leucine and HMB have been shown to stimulate muscle protein synthesis whilst HMB has also been shown to preserve muscle mass by reducing muscle breakdown, subsequently improving clinical outcomes such as wound healing, physical function and mortality44-50.
Table 2 below outlines the recommended protein requirements for older adults in health and disease41,42:
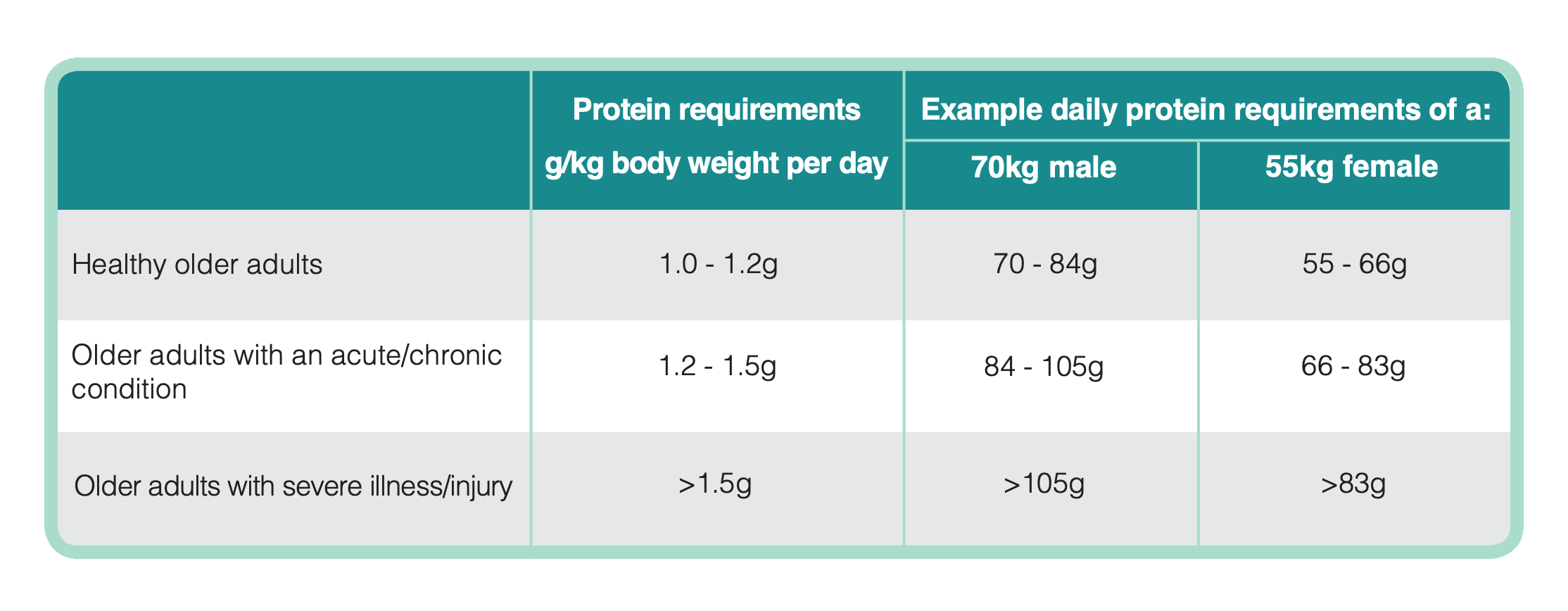
Table 2: Recommended Protein Requirements for Older Adults
ACHIEVING AN ADEQUATE INTAKE OF PROTEIN IN EVERYDAY PRACTICE
Multiple studies have indicated that 25–30 g of high-quality protein is necessary at each meal to optimally build or maintain muscle in older people and those who are unwell41,51-54. As appetite can diminish with increasing age and when unwell, this can be difficult to achieve without nutritional support and dietary advice41,55. Research shows that protein intakes among older adults, those who are unwell and those who are malnourished or at risk of malnutrition, are often inadequate42,56.
Individuals should be encouraged to eat 3-4 portions of high protein foods per day. When appetite is poor, eating three smaller meals along with snacks or milky drinks in between may help achieve nutritional requirements. Diet variety is important but good sources of protein include meat, fish, eggs, and dairy foods such as milk, yogurt and cheese. Plant-based sources of protein include soy and tofu, beans, pulses, nuts and seeds. The amino acid leucine can be found in foods such as beef, chicken, pork, dairy foods, soybeans and tofu, and in smaller amounts in eggs and seeds (e.g. pumpkin seeds).
FURTHER INFORMATION on including protein rich foods in the diet can be found on the Malnutrition Pathway website.
PROTEIN SUPPLEMENTATION
When protein intake from diet alone is insufficient, oral nutritional supplements (ONS) may be needed in addition to dietary advice, to meet requirements. ONS, particularly high protein ONS, have been shown to significantly improve protein intakes and clinical outcomes57, and may be useful to consider for patients at high risk of malnutrition and where sarcopenia is present.
The protein content of ONS can vary considerably, some have added leucine or HMB. It is important to consider the nutritional content of the product and whether it can optimally address the nutritional deficits and meet requirements and achieve the desired outcomes, dietitians are specifically skilled in this aspect of care.
ONS also provide additional energy, and the majority also provide essential micronutrients (minerals and vitamins including Vitamin D) to improve overall nutrient intakes.
When choosing the most appropriate ONS for your patient, consider individual patient needs, including flavour preferences, mouthfeel, appetite and the capacity to manage larger volumes, along with dexterity and physical and cognitive function to be able to act on the advice, and follow instructions including making up a powdered supplement for example.
FURTHER INFORMATION on oral nutritional supplements can be found on the Malnutrition Pathway website.
Vitamin D is involved in many physiological functions, including musculoskeletal health. Insufficient levels are associated with frailty and negative health outcomes58.
About half of our Vitamin D comes from diet and the remainder from the action of sunlight on the skin. Foods rich in Vitamin D include oily fish, red meat, liver, egg yolks and foods that have been fortified with Vitamin D such as some fat spreads, milks including plant-based milks and breakfast cereals.
In the UK, an over-the-counter Vitamin D supplement is recommended to provide an additional 10 micrograms (10 mcg), or 400 IU, a day for those over 65 years of age, anyone who spends a lot of time indoors, during the autumn and winter months when the level of sunlight is low, and in those who cover their skin or have dark skin. Vitamin D3 versions are better absorbed by the body. A therapeutic dose of Vitamin D may need to be prescribed in those with overt deficiency (serum 25-hydroxyvitamin D <25 nmol/L) identified from a blood test59.
Exercise in combination with diet is important to enhance uptake of protein by muscles and to enhance muscle synthesis. Individuals whose exercise is limited or who have rarely participated in exercise, should be encouraged to attend structured exercise programmes. These are usually delivered over several weeks or months and should be designed to improve strength and balance which can in turn improve mobility, maintain independence and reduce the risk of falls42,60.
Individualised regimes with supervision from appropriately qualified healthcare professionals (e.g. physiotherapists) may be needed. It is important that activity advice is tailored to the individual’s history bearing in mind that what may be a little amount of activity to one person may be a considerable amount to others.
Individuals should be encouraged to start slowly and increase the intensity and duration of their workouts over time ideally working up to 30 - 60 minutes of activity per day.
The NHS LIVE WELL website has a number of suggested activities for strengthening muscles ranging from simple chair exercises for those who are less active to more strenuous activities such as heavy gardening or lifting weight, for those who are more physically active. AGE UK also produces some useful advice on getting more active.
In all individuals, care should be taken to ensure advice is given on keeping adequately hydrated. Dehydration can cause low blood pressure, dizziness and confusion, which in turn can lead to an increased risk of falls.
Individuals should be advised to try to drink at least 6 to 8 cups of fluid every day and possibly more if individuals are losing fluid through diarrhoea, exercise or a warm environment. With the exception of alcohol, all fluids consumed count towards meeting fluid requirements - water, juice, milky drinks, tea, coffee and oral nutritional supplements.
People living with, or at risk of, frailty often take multiple medications, increasing the likelihood of side effects. Inappropriate polypharmacy may worsen underlying malnutrition as side-effects of medications (poor appetite, nausea, vomiting, constipation, diarrhoea, or low mood) contribute to a diminished food intake. In addition, antihypertensive medications have been associated with an increased risk of serious fall injuries, particularly among those who have had a previous fall61. It is important therefore that individuals at risk of frailty undergo structured medication reviews so that medications that no longer confer a benefit or pose a health risk or harm, can be rationalised or discontinued and ensure that the older adult is taking the right medications at the right doses.
Falls are a major risk for frail older adults. Fall prevention interventions can help to reduce the risk of falls and their consequences. Appropriate management of osteoporosis should also be considered in order to minimise the risk of fragility fractures.
Social isolation can contribute to frailty. Social support interventions can help to connect people with their community and provide them with emotional support. Consider the use of social prescribers to assist in accessing support to assist food purchase and meal preparation as well as identify potential challenges. The Malnutrition Pathway website includes FURTHER INFORMATION on the role of social prescribers in nutritional care.
Physical frailty is quite often accompanied by cognitive impairment62 and there is often a bi-directional relationship between the two conditions. Delirium, acute confused states, falls and functional decline are both a cause and a consequence of escalating care needs. Malnutrition risk has also been found to be linked to the progression of dementia63-65. It is important therefore to identify these issues which are common in frail individuals.
It is also important to optimise the management of any co-morbidities including long-term conditions such as COPD, cancer or heart failure - small gains made across multiple domains could help improve the QoL of the individual concerned.
Should focus on life-course progression (e.g. prefrailty, mild frailty, severe frailty) and the continuum of care (mild versus advanced care planning). Interventions need to be realistic and pragmatic with easy-to-measure outcome parameters, for example symptom management, quality of life (QoL), activities of daily living or fatigue scores39.
Goals of any intervention should be co-created and agreed with the individual taking into consideration disease stage, overall treatment / healthcare aims, and what matters to the individual. Appropriate review and follow-up need to be in place including which health or care professional is responsible for monitoring.
Advanced care planning is an important consideration, particularly for individuals with more severe frailty (CFS 7 and above), to ensure support is appropriate and acceptable as time progresses, this should be underpinned by an understanding of an individual's personal values, life goals, and preferences regarding the provision of medical care in the future.
- Frailty is not an inevitable part of ageing or disease
- With an increasingly aged population living with multiple long-term conditions, lack of action to treat or prevent frailty will result in increasing numbers of individuals being affected by frailty.
- If we fail to identify and treat frailty, we place individuals at risk of adverse outcomes including the detrimental effects on the health and quality of life of the individuals affected (and their families) and subsequent economic and clinical pressures on the health and care systems
- The NHS Long Term plan recognises that services are not consistently joined-up or responsive to the needs of people living with frailty and has outlined the need to identify and provide proactive support to older people living with frailty in the community66
- There are simple tools available to assist in the identification of frailty along with tools for identifying malnutrition and sarcopenia that are key contributory factors amenable to intervention.
- Person-centred interventions to address deficits in nutrition, physical function and activity, cognition and social engagement are key to ensuring positive outcomes
- Management should utilise nutritional and physical interventions alongside medication reviews to delay, slow or reverse frailty
- Turner G. Introduction to Frailty, Fit for Frailty Part 1. British Geriatrics Society. 2014. Available at: https://www.bgs.org.uk/resources/introduction-to-frailty
- Clegg A, et al. Frailty in elderly people. Lancet. 2013 Mar 2;381(9868):752-62.
- Boulos C, Salameh P, Barberger-Gateau P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin Nutr 2016; 35 (1): 138–143.
- Bollwein J, et al. Nutritional status according to the mini nutritional assessment (MNA®) and frailty in community dwelling older persons: a close relationship. J Nutr Health Aging 2013; 17 (4): 351–356.
- Laur C, et al. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab 2017; 42 (5): 449–458.
- Fried L, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56A(3):M146–M156.
- NIHR Dissemination Centre. Themed Review—Comprehensive Care: Older People Living with Frailty in Hospitals. National Institute of Health Research, 2017.
- Office for National Statistics. Population estimates of the population for the UK, England and Wales, Scotland and Northern Ireland: mid-2023 . www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2023 (accessed October 2024)
- NHS Confederation. Supporting People with Frailty. 12 March 2024. www.nhsconfed.org/publications/supporting-people-frailty /
- Hoogendijk EO, et al. Frailty: implications for clinical practice and public health. Lancet. 2019 Oct 12;394(10206):1365-1375.
- Kingston A, et al. Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age and Ageing. 2018; 47 (3): 374-380.
- Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019 Feb 14; 12:23-30.
- Health Education England, NHS England and Skills for Health. Frailty: A framework of core capabilities. 2018. www.skillsforhealth.org.uk/wp-content/uploads/2021/01/Frailty-framework.pdf
- Roberts S, Collins P, Rattray M. Identifying and Managing Malnutrition, Frailty and Sarcopenia in the Community: A Narrative Review. Nutrients. 2021; 13(7):2316.
- Dodds R, Sayer AA. Sarcopenia and frailty: new challenges for clinical practice. Clin Med (Lond). 2016 Oct;16(5):455-458.
- Ni Lochlainn M, et al. Nutrition and Frailty: Opportunities for Prevention and Treatment. Nutrients. 2021; 13(7):2349.
- Cruz-Jentoft A, Sayer A. Sarcopenia. The Lancet. 2019; 393(10191): 2636-2646.
- Beaudart C, et al. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients. 2019; 11(12): 2883.
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018; 14(9): 513-537.
- Verlaan S, et al. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults-A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2017 May 1;18(5):374-382.
- Stratton R, Smith T, Gabe S. Managing malnutrition to improve lives and save money. Redditch: British Association for Parenteral and Enteral Nutrition. 2018. Available at: www.bapen.org.uk/pdfs/reports/mag/managing-malnutrition.pdf
- Wilson N, Mullaney W. Frailty and nutrition. Br J Community Nurs. 2024 Mar 2;29(3):118-123.
- Office for Health Improvement and Disparities (OHID). Obesity Profile: short statistical commentary May 2024. https://www.gov.uk/government/statistics/update-to-the-obesity-profile-on-fingertips/obesity-profile-short-statistical-commentary-may-2024) (accessed October 2024).
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010; 58: 681–7.
- Rockwood K, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005 Aug 30;173(5):489-95.
- Rockwood K, Theou O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can Geriatr J. 2020 Sep 1;23(3):210-215.
- NHS England. Identifying frailty. https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/frailty-risk-identification/ (accessed October 2024).
- Cruz-Jentoft A, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39(4):, 412-423.
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc.2013; 14(8), pp.531-532.
- Spiegowski D, Metzger L, Jain A, et al. The Utility of Grip Strength as a Simplified Measure of Frailty in the Older Adult in the Preoperative Clinic. Cureus 2022;14(9): e28747.
- Cruz-Jentoft A, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48(1): 16-31.
- Centers for Disease Control and Prevention. Stop Elderly Accidents Deaths and Injuries – 30 Second Chair Stand Assessment Leaflet.2017. www.cdc.gov/steadi/media/pdfs/STEADI-Assessment-30Sec-508.pdf (accessed October 2024).
- Elia M, editor. The ‘MUST’ report. Nutritional screening for adults: a multidisciplinary responsibility. 2003. Redditch, UK, BAPEN.
- Cederholm T, et al., GLIM criteria for the diagnosis of malnutrition. A consensus report from the global clinical nutrition community. Clinical Nutrition. 2019; 38 : 1 – 9.
- Dedeyne L, et al. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging. 2017 May 24; 12:873-896.
- British Geriatrics Society. Frailty: what’s it all about? 23 May 2018. www.bgs.org.uk/resources/frailty-what%E2%80%99s-it-all-about
- Morley J, et al. Frailty Consensus: A Call to Action. Journal of the American Medical Directors Association. 2013;14(6):392-3973
- British Geriatric Society (BGS). Comprehensive Geriatric Assessment Toolkit for Primary Care Practitioners. 2019. www.bgs.org.uk/cgatoolkit
- Holdoway A, et al. Individualised Nutritional Care for Disease-Related Malnutrition: Improving Outcomes by Focusing on What Matters to Patients. Nutrients. 2022;14(17): 3534.
- Travers J et al. Delaying and reversing frailty: a systematic review of primary care interventions. British Journal of General Practice 2019; 69 (678): e61-e69.
- Bauer J, Biolo G, Cederholm T et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013; 14 (8): 542–559.
- Deutz NE, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. 2014; 33: 929-36. 21.
- Baum JI, Kim IY, Wolfe RR. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients. 2016 Jun 8;8(6):359.
- Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem 1997; 8:300-311.
- Wilkinson DJ, et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013; 591(11):2911-2923.
- Eley HL, et al. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 2008; 295: E1409-1416.
- Sanz Paris A et al. J Nutr Health Aging 2018;22(6):664-675
- Ekinci O et al. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients With Hip Fracture: A Randomized Controlled Study. Nutr Clin Pract. 2016; 31(6):829-835
- Cramer JT et al. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J Am Med Dir Assoc. 2016;1044-1055
- Deutz NE, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. 2014; 33: 929-36. 21.
- Paddon-Jones and Leidy. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014; 17:5–11.
- Deutz and Wolfe. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. 2013; 32:309–13.
- Mamerow, et al. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr. 2014; 144:876–80.
- Luiking, et al. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: a randomized controlled trial. Nutr J. 2014; 13:9.
- Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People. 2015 Jun;27(5):29-35.
- Morris S, Cater JD, Green MA, Johnstone AM, Brunstrom JM, Stevenson EJ, Williams EA, Corfe BM. Inadequacy of Protein Intake in Older UK Adults. Geriatrics (Basel). 2020 Feb 12;5(1):6
- Cawood A, et al. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012 Apr;11(2):278-96
- Buchebner D, et al. Association Between Vitamin D, Frailty, and Progression of Frailty in Community-Dwelling Older Women. J Clin Endocrinol Metab. 2019 Dec 1;104(12):6139-6147.
- National Institute for Health and Care Excellence (NICE). Vitamin D deficiency in adults 2021. https://cks.nice.org.uk/topics/vitamin-d-deficiency-in-adults
- Kumar V, et al. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009; 106(6): 2026-2039.
- Tinetti ME, et al. Antihypertensive Medications and Serious Fall Injuries in a Nationally Representative Sample of Older Adults. JAMA Intern Med. 2014;174(4):588–595.
- Ma L, Chan P. Understanding the Physiological Links Between Physical Frailty and Cognitive Decline. Aging Dis. 2020 Mar 9;11(2):405-418.
- Haveman-Nies A, et al. Fluid intake of elderly Europeans. The Journal of Nutrition, Health and Aging. 1997;1:151–5. 12.
- Martin MA, et al. Presence of malnutrition and risk of malnutrition in institutionalized elderly with dementia according to the type and deterioration stage. Nutr Hosp. 2012;27:434–40.
- Jesus P, et al. Nutritional assessment and follow-up of residents with and without dementia in nursing homes in the Limousin region of France: a health network initiative. J Nutr Health Aging. 2012; 16:504–8
- NHS Long Term Plan. 2019. www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf
This factsheet has been produced with an unrestricted educational grant from Abbott
Resources
A selection of publications for use by healthcare professionals, patients and carers are available in the resources section of the website.
Support for Patients & Carers
A number of resources are available that have been developed to support patients and carers.
Specific support for common conditions
A number of resources are available that have been developed to assist healthcare professionals supporting patients at risk of malnutrition as a result of a specific condition. These include:
Further Information
We can be contacted regarding the malnutrition pathway materials and website